
The algorithm involves initial T-cell receptor incision circles (TRECs) testing of the blood spot specimen routinely collected after birth ≥ 24 hours. Hawaii follows the testing algorithm used by the Oregon State Public Health Laboratory (OSPHL), Hawaii’s contracted NBS laboratory. To ensure the babies born in Hawaii have access to the life-saving treatment options, a partnership between Kapi’olani Medical Center for Women and Children, the University of California at Los Angeles Mattel’s Children’s Hospital, and the Hawaii Department of Health has been created.Long-term treatment is a bone marrow transplant or gene therapy.Common infections and vaccines are life-threatening without treatment. SCID occurs once in every 54,000 births in the United States. If your baby’s newborn screening result for severe combined immunodeficiency (SCID) was out of the normal range, your baby’s doctor or the state screening program will contact you to arrange for your baby to have additional testing.NBS does not detect all cases of SCID in newborns.Families and infants diagnosed with SCID should receive immediate confirmatory testing and a Pediatric Immunologist evaluation with counseling.The Hawaii Newborn Screening Program can assist you with the coordination of the follow-up to confirm a diagnosis of SCID in the newborn. SCID is a paediatric emergency and is life. Affected children are born without cellular and humoral immunity and are therefore highly susceptible to bacterial, viral, fungal and opportunistic infections. SCID is the most severe form of inherited primary immunodeficiency (Lipstein 2010).
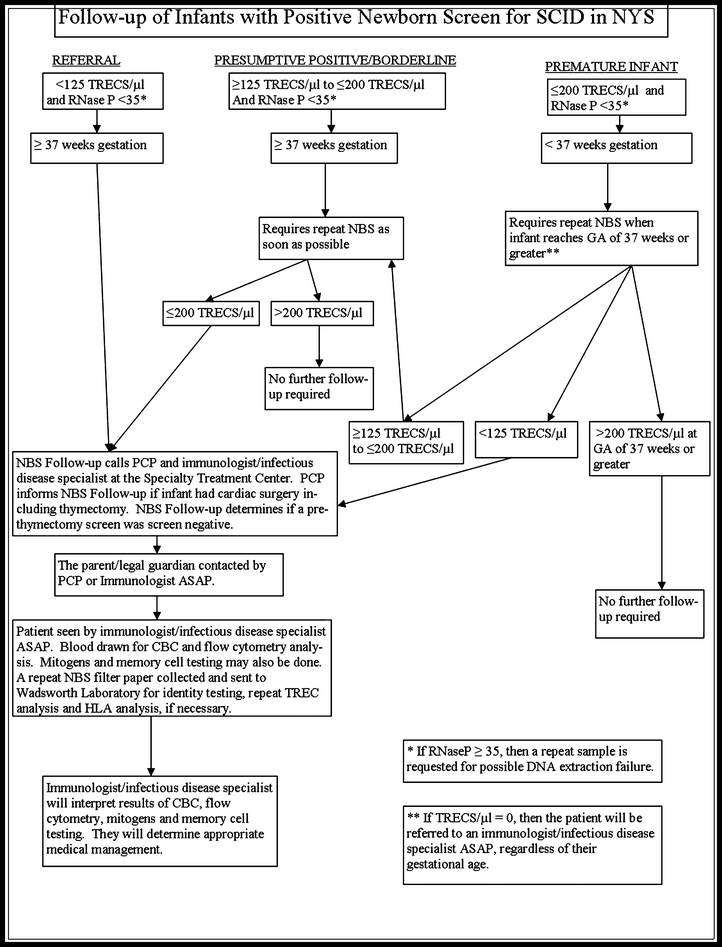
Diagnosis of SCID needs to be confirmed with additional diagnostic tests and a Pediatric Immunologist evaluation. A White Paper on Newborn Screening for SCID in Europe. The T-cell receptor excision circle test. DO NOT base a diagnosis of SCID on a positive NBS result. Newborn screening for severe combined immunodeficiency, the most profound form of primary immune system defects, has long been recognized as a measure that would decrease morbidity and improve outcomes by helping patients avoid devastating infections and receive prompt immune-restoring therapy. Severe Combined Immunodeficiency (SCID) is on the state newborn screening (NBS) panel as of March 1, 2015.Sample Abnormal and Normal SCID Newborn Screen Report SCID newborn screening is already standard practice in many countries including the USA, Canada, Norway, Spain and New Zealand.SCID fulfils the internationally.This path was initiated by the United States in 2010, with the Department of Health and Human Services recommending SCID for uniform screening. SCID is on the NBS panel as of March 1, 2015. Newborn screening for severe combined immunodeficiencies (SCID) is slowly but surely becoming a reality in many countries. With the support of the Hawaii community-based SCID Task Force, the Hawaii Newborn Metabolic Screening Program Advisory Committee recommended the addition of SCID to the state newborn screening (NBS) panel. SCID Newborn Screening Incidence Treatment hematopoietic stem cell transplant (HSCT), enzyme replacement, gene therapy Diagnosis Missed - often due to normal appearance in the newborn period - results in death in the first year of life Timing - optimizes treatment outcome and reduces morbidity < 3. NEWBORN SCREENING FOR SEVERE COMBINED IMMUNODEFICIENCY (SCID) BACKGROUND


 0 kommentar(er)
0 kommentar(er)
